Background:
Recent data from the field of autoimmune platelet antibodies in vaccine-induced immune thrombotic thrombocytopenia (VITT) suggests that antibodies with restricted clonality may be associated with more severe outcomes. Warm autoimmune hemolytic anemia (WAIHA) is an IgG-mediated red blood cell (RBC) disorder. The goal of this study was to investigate the clonality of anti-RBC IgG antibodies in WAIHA, and to assess if clonal restriction was associated with more severe disease course and outcomes.
Methods:
15 patients with WAIHA were evaluated. All patients had proven IgG positivity in direct antiglobulin testing (DAT, or “Coombs” test). RBC eluates were prepared and both the patient plasma sample, as well as the RBC eluate, were subject to liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF MS). Based on the number of antibodies seen in the RBC eluate, patients were classified into mono/oligoclonal vs. polyclonal antibody WAIHA groups
Assessment of response to treatment was based on international consensus criteria: complete (CR; normal Hb or Hb ≥12 g/dL, with resolution of hemolysis); partial (PR; Hb >10 but <12 g/dL or Hgb >12 g/dL without resolution of hemolysis); or no response (neither CR nor PR).
Results:
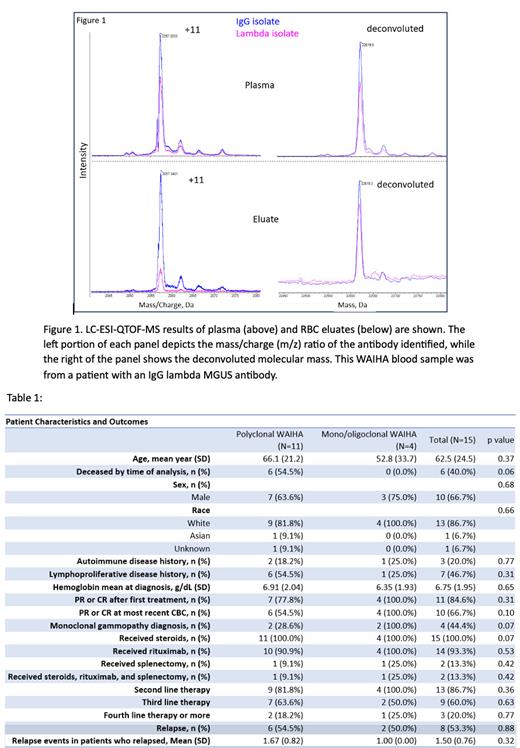
Eleven of the 15 patients (73%) were found to have polyclonal antibodies, while four patients (27%) had mono/oligoclonal WAIHA antibodies. Of these four patients with restricted clonality, two patients had monoclonal antibodies (Figure 1 shows one of the monoclonal WAIHA antibodies identified). There was no significant difference in demographic composition, clinical findings, or treatment received in both groups (Table 1). 66.7% (n=10) of patients were male, and the mean age (SD) was 62.5 years (24.5). Two pediatric patients, ages 10 and 16, were included. Concomitant lymphoproliferative disease was present in 46.7% of the patients. The three most common treatments were steroids (100%), rituximab (93.3%), and splenectomy (13.3%). 40.0% of patients had died at the time of this analysis with no difference between the groups.
The number of treatments received were similar between the mono/oligoclonal and polyclonal groups. After the first line of treatment, 100% and 77.8% of the mono/oligoclonal and polyclonal groups respectively achieved PR or CR (P=0.31). Additionally, 100% of the mono/oligoclonal group and 54.5% of the polyclonal group were in PR or CR by their most recent CBC (P=0.10). 50.0% and 54.5% of the mono/oligoclonal group and polyclonal groups respectively relapsed at least once (P=0.25). In those who relapsed, there was an average of 1.0 relapse events in the mono/oligoclonal group compared to 1.7 relapse events in the polyclonal group (P=0.32).
Conclusion:
This study demonstrates, for the first time, that monoclonal and oligoclonal antibodies can mediate warm autoimmune hemolytic anemia. While these data suggest no difference in clinical outcomes or disease course between patients with mono/oligoclonal vs. polyclonal anti-RBC antibodies, a key limitation is the small sample size.
Disclosures
Murray:Eastman Kodak: Patents & Royalties. Pabmanabhan:Versiti Inc: Patents & Royalties; Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees; Retham Technologies: Current equity holder in private company, Patents & Royalties; Mayo Clinic: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal